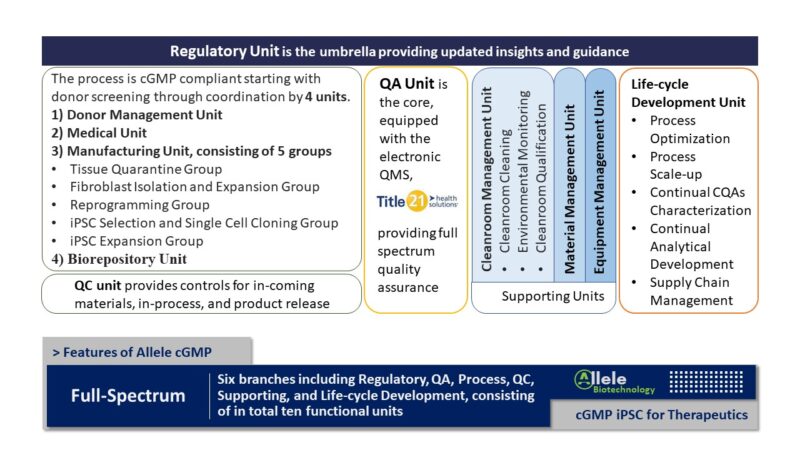
The Allele Biotech cGMP Manufacturing team has been at the forefront of cGMP-compliant iPSC expansion since 2016. Our well-developed and rigorously validated cGMP iPSC expansion processes are designed to consistently scale up iPSC cell production, ranging from millions to billions of cells, tailored to the specific needs of your projects.
These expanded cells can be harvested and formulated for cryopreservation or further processing, offering customization options for concentration, formulation, container selection, and packaging units.
While utilizing Allele’s iPSC lines ensures seamless integration into our cGMP production, our development team can efficiently facilitate the incorporation of any qualified non-Allele iPSC lines into our cGMP production with a brief assessment and necessary adaptations.
After your starting iPSC source undergoes our cGMP-compliant iPSC expansion process, first passing all in-process controls and final release testing, subsequently undergoing rigorous process and analytical quality assurance reviews, we will provide a certificate of analysis for the cGMP lot. This certificate will distinctly outline the cGMP compliance, including all supporting details.
An additional significant advantage of conducting cGMP iPSC expansion at Allele Biotech is that we will provide an extensive Standard Operating Procedure (SOP) detailing the iPSC expansion process. This invaluable SOP can serve as a crucial resource for your internal training and any potential tech transfer endeavors in the future.
