Selecting an iPSC line can be a complex process. The questions outlined above represent the essential considerations that can significantly assist in your decision-making process. For a more comprehensive guide to iPSC selection, please download our iPSC Selection Guide or reach out to us via email at info@allelebiotech.com.

Which cGMP or clinical-grade iPSC line are you using?
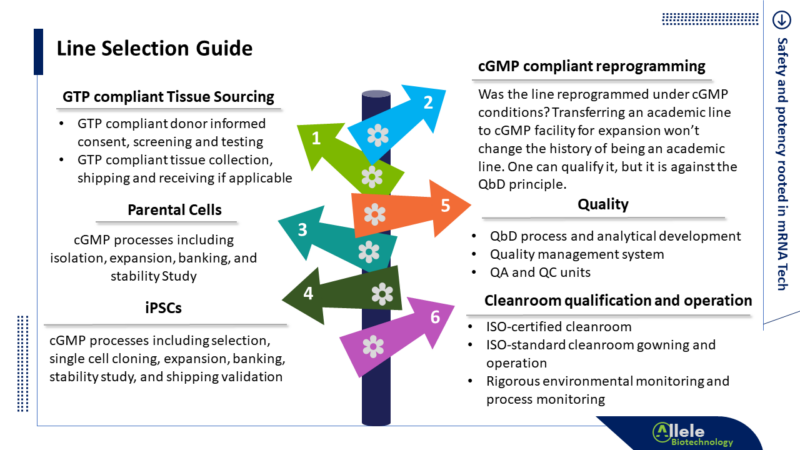
We trust that clinical iPSC users have already reviewed and confirmed the informed consent and other good tissue practice (GTP) compliance-related information for your iPSC line. If not, we highly recommend adding this essential step to the top of your to-do list right away.
Allele Biotech’s cGMP iPSC line is developed through a comprehensive end-to-end cGMP process, beginning with donor recruitment, progressing through donor screening, obtaining donor consent, collecting tissue, and seamlessly transitioning into cGMP manufacturing.

Have you had the opportunity to compare multiple iPSC lines before making a commitment?
Given the limited availability of cGMP or clinical-grade iPSC lines, conducting comparison studies before settling on a line may seem like a luxury. However, this perception of “limited availability” is misleading. Leveraging the time-effectiveness and consistency of Allele’s cGMP reprogramming, powered by our patented mRNA-based reprogramming technology, we can establish new iPSC lines under full cGMP in less than three months. Our validated capacity allows us to work on up to 15 iPSC lines simultaneously. Whether you choose from our off-the-shelf iPSC line library or opt for our customized cGMP iPSC Reprogramming service, you can have several iPSC lines for your comparison studies as soon as within a couple of weeks and no later than three months.

Have you evaluated the stability of the iPSC line you've committed to?
Here are our suggestions: consider examining attributes such as the doubling time or other proliferation-related characteristics; observe morphological features, including the size, compactness, and edges of the iPSC cell patches, the size of individual iPSCs, the frequency and degree of the presence of spontaneous differentiation, as well as the percentage of overgrowth areas; monitor variations in the percentage of iPSC surface marker-positive cell populations and identify any subpopulations based on expression levels.
Also, it is essential to tackle the more challenging task of assessing genome stability and emphasizing the efficiency of your differentiation protocols early in the iPSC-based cell therapy program development.
These activities should be conducted over an extended period and passage numbers. Observations of significant differences among the iPSC lines generated by various reprogramming technologies are common in our experience. Allele’s unique mRNA reprogrammed lines have always been a winner judged by pharmaceutical and biotech companies who have done side-by-side comparisons.
Your differentiation protocol sets the stage for cGMP production, where consistency and process controls are paramount. The stability of your starting cell source represents one of the most critical control steps in this process.