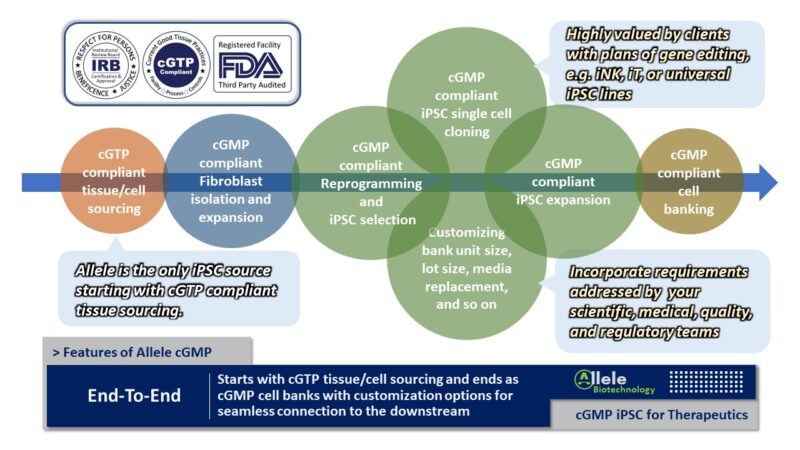
There is a common misconception in the iPSC field that good cGMP iPSC lines are in limited supply and it is challenging to develop new ones. Countering that notion, Allele possesses a unique advantage in shifting that perception by harnessing Allele’s mRNA reprogramming technology. This reprogramming approach typically yields lines that are scientifically and operationally validated for safety, efficiency, and consistency. Currently, we maintain an extensive off-the-shelf cGMP iPSC line library featuring 24 healthy donors with diverse demographics. The expansion of this library is already in motion, with a pace set for adding 24 new lines per year. Off-the-Shelf cGMP iPSC Lines
With the routine cGMP iPSC generating capacity comes Allele’s Custom cGMP iPSC Line Development for partners and clients. Allele stands out globally with over ten years of continuous cGMP process development with a track record of zero failures in reprogramming.
There are unique benefits of putting in the extra effort to create a customized cGMP iPSC line or library for a particular program. These include securing donor exclusivity or even donor selectivity based on health status or genetic background to be more aligned with your project’s requirements.